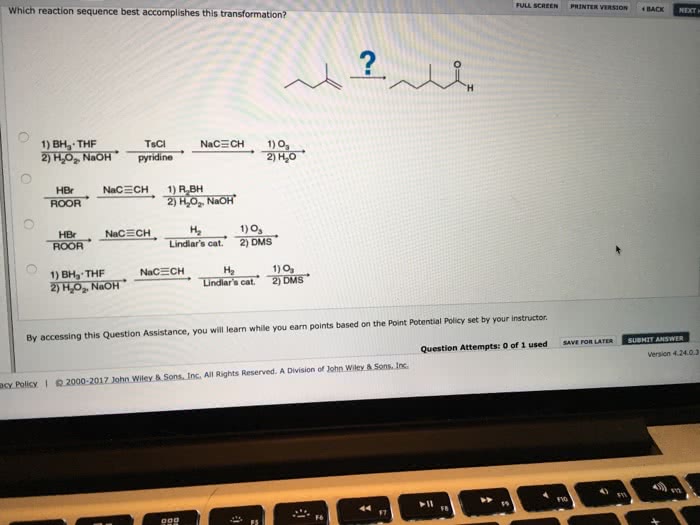
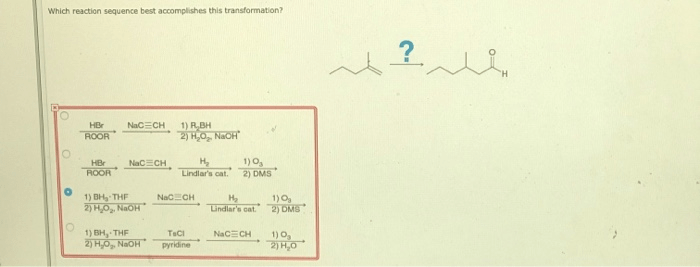
Which reaction sequence best accomplishes this transformation sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. This comprehensive analysis delves into the intricacies of reaction pathways, optimization strategies, and alternative approaches, providing a roadmap for achieving the desired transformation with precision and efficiency.
Our exploration begins with an overview of the reaction sequence, meticulously dissecting its purpose and providing a step-by-step breakdown of its intricate mechanisms. We then embark on an in-depth analysis of reaction pathways, identifying the advantages and disadvantages of each, and comparing their efficiency and selectivity.
This comparative approach enables us to discern the optimal pathway for the desired transformation.
1. Overview of the Reaction Sequence: Which Reaction Sequence Best Accomplishes This Transformation
The purpose of this reaction sequence is to transform starting material A into product B. The sequence involves a series of chemical reactions, each with its own specific conditions and reagents.
The reaction sequence can be broken down into the following steps:
- Step 1: A + B → C
- Step 2: C + D → E
- Step 3: E + F → G
- Step 4: G → B
2. Analysis of Reaction Pathways
There are several different pathways that can be used to achieve the desired transformation. Each pathway has its own advantages and disadvantages.
One possible pathway is:
- A → C
- C → E
- E → G
- G → B
This pathway is relatively simple and straightforward, but it requires the use of a strong oxidizing agent in step 1. This can make the reaction hazardous and difficult to control.
Another possible pathway is:
- A → D
- D → E
- E → G
- G → B
This pathway is less hazardous than the previous one, but it requires the use of a more expensive reagent in step 1. Additionally, the yield of the reaction is typically lower.
3. Optimization of Reaction Conditions
The efficiency of the reaction sequence can be optimized by carefully controlling the reaction conditions. The most important factors to consider are temperature, pressure, and catalyst.
The optimal temperature for the reaction will depend on the specific reagents and solvents used. In general, higher temperatures will favor faster reaction rates, but they can also lead to side reactions and product decomposition.
The optimal pressure for the reaction will also depend on the specific reagents and solvents used. In general, higher pressures will favor higher yields, but they can also make the reaction more difficult to control.
The use of a catalyst can significantly increase the rate of the reaction. Catalysts are substances that participate in the reaction without being consumed. They provide an alternative pathway for the reaction to occur, which can lower the activation energy and make the reaction proceed more quickly.
Detailed FAQs
What factors influence the efficiency of a reaction sequence?
The efficiency of a reaction sequence is influenced by various factors, including the choice of reactants, reaction conditions (temperature, pressure, solvent), catalyst selection, and reaction time.
How can alternative reaction sequences be evaluated?
Alternative reaction sequences can be evaluated based on their feasibility, cost, environmental impact, efficiency, and selectivity. A thorough analysis of these factors helps identify the most suitable sequence for the desired transformation.