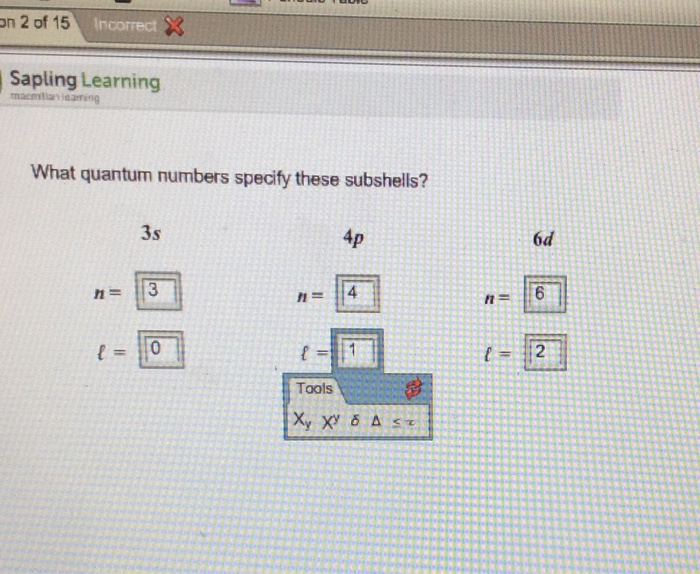
Beginning with the question “what quantum numbers specify these subshells 4d,” this article delves into the fascinating world of quantum mechanics, unraveling the mysteries of electron subshells and the quantum numbers that define them. Prepare to embark on a journey that will illuminate the intricate dance of electrons within atoms, revealing the profound influence of quantum numbers on their behavior and properties.
Quantum numbers, the cornerstone of describing electron subshells, provide a precise language for understanding the intricate world of atomic structure. This article will explore the three fundamental types of quantum numbers—principal, azimuthal, and magnetic—and their significance in characterizing the 4d subshell.
We will delve into the energy level, shape, and orientation of this subshell, deciphering the quantum numbers that govern its unique properties.
Quantum Numbers: What Quantum Numbers Specify These Subshells 4d
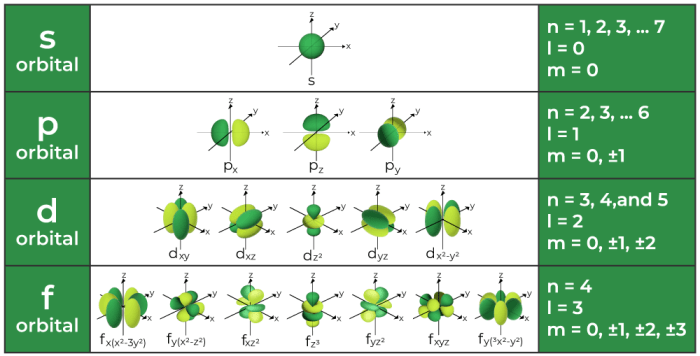
Quantum numbers are a set of four numbers that describe the state of an electron in an atom. They are:
- Principal quantum number (n): This number describes the energy level of the electron.
- Azimuthal quantum number (l): This number describes the shape of the electron’s orbital.
- Magnetic quantum number (ml): This number describes the orientation of the electron’s orbital in space.
- Spin quantum number (ms): This number describes the spin of the electron.
4d Subshell
The 4d subshell is a set of orbitals that have the same principal quantum number (n = 4) and azimuthal quantum number (l = 2). It is located in the fourth energy level of an atom.
The 4d subshell can hold up to 10 electrons.
Quantum Numbers for 4d Subshell, What quantum numbers specify these subshells 4d
The quantum numbers that specify the 4d subshell are:
| Quantum Number | Value |
|---|---|
| Principal quantum number (n) | 4 |
| Azimuthal quantum number (l) | 2 |
Electron Configuration
The electron configuration of an atom is a notation that describes the arrangement of electrons in the atom’s orbitals. The electron configuration of an atom with electrons in the 4d subshell can be written as:
[Core] 4d x
where [Core] represents the electron configuration of the atom’s core electrons and x is the number of electrons in the 4d subshell.
Chemical Properties
Elements with electrons in the 4d subshell are typically transition metals. Transition metals are characterized by their ability to form multiple oxidation states and their tendency to form complexes with other atoms.
Questions and Answers
What is the significance of quantum numbers in describing electron subshells?
Quantum numbers provide a precise and comprehensive language for characterizing electron subshells. They specify the energy level, shape, and orientation of subshells, enabling a detailed understanding of electron behavior within atoms.
How many quantum numbers are required to specify an electron subshell?
Three quantum numbers are necessary to uniquely specify an electron subshell: the principal quantum number (n), the azimuthal quantum number (l), and the magnetic quantum number (ml).
What is the 4d subshell, and how is it characterized?
The 4d subshell is a specific subshell within the fourth energy level (n=4) of an atom. It has an azimuthal quantum number (l) of 2, indicating a d-orbital shape. The 4d subshell can accommodate up to 10 electrons.